unigold hiv test package insert|10 day hiv test accuracy : trade All testing must be carried out in accordance with the Uni-Gold™ . web2 de fev. de 2024 · Dans cet article, nous parlons de la nouvelle adresse Streamonsport en Février 2024, qui est considéré comme le meilleur site de streaming football gratuit. Nous discuterons de son fonctionnement, .
{plog:ftitle_list}
01:30. 02:00. 02:30. 03:00. 03:30. 04:00. 04:30. 05:00. 05:30. 06:00. 06:30. 07:00. 07:30. 08:00. 08:30. 09:00. 09:30. 10:00.
Uni-Gold™ Recombigen® HIV-1/2 was designed as a rapid immunoassay and is intended to detect antibodies to HIV-1 and/or HIV-2 in human serum, plasma and whole blood .
All testing must be carried out in accordance with the Uni-Gold™ .Manufacturer: Trinity Biotech. Tradename: Uni-Gold™ Recombigen® HIV-1/2. Indication: Indicated as a single use rapid immunoassay for the qualitative detection of antibodies to HIV .HIV-1/2 Test Results in 10 Minutes. Complete Sample Flexibility: Whole Blood-Serum-Plasma. Easy to Use: Three Simple Steps. Add Patient Sample. Add Wash Solution. Read Results.
Uni-GoldTM Recombigen® HIV-1/2 Third Generation Rapid HIV-1/2 Test Results in 10 Minutes BENEFITS Uni-GoldTM Recombigen . For full instructions, please refer to the package insert. Trinity Biotech USA 2823 Girts Road Jamestown, NY 14701 Tel: 800-325-3424 [email protected]
When multiple rapid HIV test are available, this test can be used in appropriate multi-test algorithms. Determine HIV-1/2 Ag/Ab Combo is not intended for newborn screening or for use with cord .The INSTI HIV-1/HIV-2Antibody Test is a single use, rapid, in vitro qualitative immunoassay for the detection of antibodies to Human Immunodeficiency Virus Type 1 and/or Type 2 (HIV-1/HIV-2) in .The OraQuick® Rapid HIV-1 Antibody Test is a single-use, qualitative immunoassay to detect antibodies to Human . The OraQuick ® Rapid HIV -1 Antibody Test is a point -of-care test to aid in the diagnosis of infection with HIV -1. Read this package insert completely before using the product. Follow the instructions carefully. Not doing so
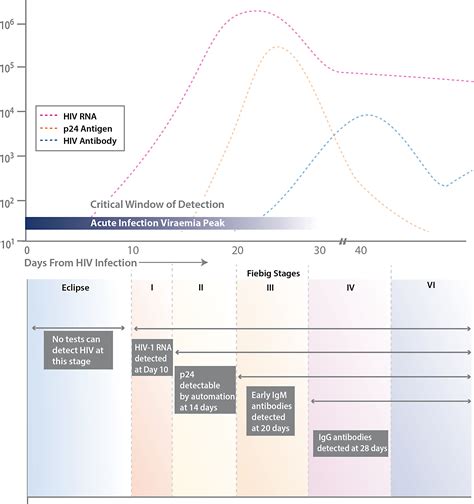
The first FDA approved rapid point-of-care test that detects both HIV-1/2 antibodies and free HIV-1 p24 antigen. This antigen/antibody test (4th generation) has the ability to identify HIV earlier than antibody-only tests (2nd and 3rd generation). 1 It enables health care providers to diagnose HIV infection earlier allowing individuals to seek medical care sooner.Determine™ HIV-1/2 Test (20 tests) 7D2346. 7D2342: Determine™ HIV-1/2 Test (100 tests) 7D2347: 7D2343: Determine™ HIV-1/2 Test Set (100 tests and accessories) 7D2343SET: Chase Buffer (for whole blood) 7D2243: 7D2243: EDTA Capillary Tubes (x100) 7D2227. 7D2222
HIV 1/2 Antibody Test. Bioline™ HIV 1/2 3.0 test is an immunochromatographic assay for the differential and qualitative detection of all isotypes (IgG, IgM, IgA) antibodies specific to HIV-1 including subtype O and HIV-2 simultaneously, in human serum, plasma or whole blood. Product not available in all countries.
of-care test to aid in the diagnosis of infection with HIV-1 and HIV-2. This test is suitable for use in multi-test algorithms designed for the statistical validation of rapid HIV test results.HIV-1 AND HIV-2 POSITIVE CONTROLS WILL PRODUCE A WEAKLY REACTIVE RESULT IN THE INSTI® HIV-1/HIV-2 ANTIBODY TEST. THE HIV NEGATIVE CONTROL WILL PRODUCE A NONREACTIVE RESULT IN THE INSTI ® HIV-1/HIV-2 ANTIBODY TEST. 1. Use a new pipette for each Control sample collection. Take the pipette and lightly depress the top bulb. 2. Insert .Uni-Gold™ HIV is a single use rapid immunoassay, for the qualitative detection of antibodies to HIV-1 and HIV-2 in serum, plasma and whole blood (venous and fingerstick). Uni-Gold™ HIV is intended for use in point of care settings as an aid in diagnosis of HIV-1 and HIV-2 infection.The Uni-Gold™ range of rapid point-of-care test devices consist of high-quality, single use immunoassays to aid in the diagnosis of HIV. The ease of use and robustness of the products ensures they can be reliably used in diverse settings including clinical laboratories, point-of-care testing centres. All products in the range offer:
approval for our new HIV screening product, TrinScreen HIV. This product has been a strategic priority for Trinity Biotech over several years and this approval is a very positive development for the company’s future. We have already earned a strong reputation in the HIV testing market in Africa with our HIV confirmatory test, Uni-Gold HIV.
rapid hiv test accuracy chart
The INSTI™ HIV-1/HIV-2 Antibody Test can be used as an aid in the diagnosis of -1 andHIV/or HIV-2 infection in point of care settings. Using a rapid HIV test provides an opportunity to identify more
positive for HIV-1 p24 antigen and negative for anti-HIV-1 and anti-HIV-2 antibodies. The test is suitable for use in multi-test algorithms designed for the statistical validation of rapid HIV test results. When multiple rapid HIV test are available, this .Uni-Gold™ Recombigen® HIV Rapid Test Kits, Trinity Biotech. 1206530. 12009-002PK 64.83 USD. 12009-002. Uni-Gold™ Recombigen® HIV Rapid Test Kits, Trinity Biotech. This CLIA-waived single-use rapid immunoassay is designed for the qualitative detection of HIV-1 antibodies in serum, plasma, and whole blood.Page 1 of 7 - EN. Uni-Gold™ Recombigen® HIV-1/2 1206506 the test region of the device adjacent to the word ‘Test’. Per le altre lingue. Read this package insert completely before using the .
As noted in the package insert, clinical studies have shown that the OraQuick In-Home HIV Test has an expected performance of approximately 92% for test sensitivity (i.e., the percentage of .
The 4 th generation Determine™ HIV Early Detect test detects more acute infections compared to 2 nd and 3 rd generation tests. This helps close the window period and enables increased case finding at a time when individuals are highly infectious. . *Please refer to the Product Insert for the analytical sensitivity of HIV-1 p24 antigen .Aptima HIV-1 Quant Dx Assay 4 AW-18107-001 Rev. 001 General Information Aptima® Warnings and Precautions A. For in vitro diagnostic use only. B. The Aptima HIV-1 Quant Dx assay is not intended for use as a screening test for the presence of HIV-1 in donated blood. C. To reduce the risk of invalid results, carefully read the entire package .Uni-Gold HIV test is a single use rapid immunoassay, for the qualitative detection of antibodies to HIV-1 and HIV2 in serum, plasma and whole blood (venipuncture and fingerstick) Intend Use: Uni-Gold HIV test is intended for use in point-of-care settings as an aid in diagnosis of HIV-1 and HIV-2 infection. Technical specifications:f) 1 Package Insert Materials required and available as an accessory to the kit Uni-Gold™ Recombigen® HIV Controls Kit. Catalog number 1206530. Each pack of Kit Controls contains; HIV-1 Positive Control, 1 vial (red cap), (0.5ml), HIV-2 Positive Control, 1 vial (green cap), and Negative Control, 1 vial (black cap) (0.5ml) and a package insert.
2 Refer to product insert for details. REQUEST MORE INFORMATION CONTACT SALES TEAM CONTACT TECHNICAL SUPPORT. Test for HIV in 3 Easy Steps with STAT-PAK ® STAT-PAK ® HIV Test: Rapid HIV Test in 3 Easy Steps This video will show you how to use Chembio’s STAT-PAK ® HIV 1/2 Assay. Product Information. Information Type: Product Detail:The INSTI HIV-1/HIV-2 Antibody Test is considered a 3rd generation screening test since it is designed to detect antibodies only that are generated in response to HIV infection. 4th generation tests detect both antibodies and p24 antigen, as p24 antigen is present earlier in infection but only for a limited time.Uni-Gold™ HIV test is a single reagent assay for the detection of antibodies to human immunodeficiency virus types 1 and 2 in serum, plasma or wholeblood. Location: USA; . Uni-Gold™ HIV Complete. Location: Not available in . Our Products; Point of Care; Infectious Disease; Haemoglobins; Autoimmune; Clinical Chemistry; Antibodies & Proteins;
HIV-1/HIV-2 Antibody Test Kit distinct blue spot at the location of the control spot and, in the case that HIV antibodies are present in . Read the entire Package Insert prior to beginning the test procedure. Complete conformance with the test procedure is necessary to ensure accurate results. 2. Before performing testing, operators must read .The HIV 1.2 Rapid Test (Whole Blood) for self-testing has been compared to a leading commercial HIV ELISA kit using clinical specimens. The results show that the relative sensitivity of the HIV 1.2 Rapid Test (Whole Blood) for self-testing (whole blood) is >99.9% and the relative specificity is 99.7%.This test is suitable for use in multi-test algorithms designed for the statistical validation of rapid HIV test results. When multiple rapid HIV tests are available, this test should be used in appropriate multi-test algorithms . Uni-Gold Recombigen HIV-1/2 Trinity Biotech: N/A: For use in point of care settings as an aid in diagnosis of .
is oraquick hiv test accurate
Resultado da Clique na desenhos para colorir Freddy e Bonnie Animatronics para ver a versão para imprimir ou colori-la online (compatível com iPad e tablets .
unigold hiv test package insert|10 day hiv test accuracy